TITLE : Hetero-modal encoder for generation of coherent hypoperfused volume across MRI sequences
Keywords: deep learning, multi-modal imagery, weekly supervised training, modality fusion, machine learning, deep tech, neuroimaging, precision medicine, stroke
Period: from January-april 2026 onwards (flexible depending on candidate profile), 5-6 months
Scientific leaders : Hichem Maaref et V. Vigneron (IBISC)
Partners : IBISC (universit´e Evry-Paris-Saclay), centre hospitalier sud-francilien , WILLIS-AI
funding : Maturation project FAUST led by SATT Paris-Saclay
Basic AI and Data Science : machine learning theory, high-dimensional statistics, imagery, uncertainty, information theory
Specialized ML and AI : medical imaging, MRI preprocessing Duration : 5 to 6 months, starting between January and April 2026 funding : Maturation project FAUST led by SATT Paris-Saclay Location : IBISC lab
Application domain : precision medicine
Subject
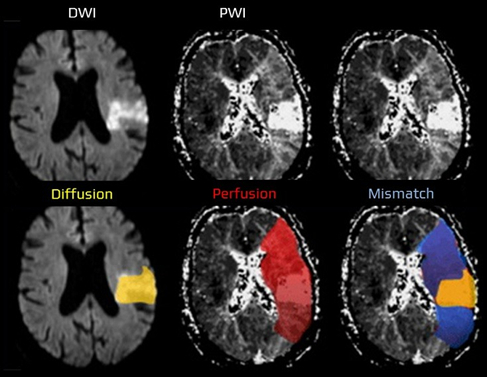
Hypoperfusion in acute ischemic stroke indicates a hemodynamic disturbance visible across multiple MRI contrasts [Forkert et al., 2013]. Each modality provides complementary in- formation: susceptibility-weighted imaging (SWAN/SWI) and phase highlight venous de- oxygenation, time-of-flight (TOF) angiography captures arterial inflow and distal signal loss, diffusion-weighted imaging (DWI) shows early cytotoxic injury, and FLAIR reveals hyperin- tense vessels and edema [Mittal et al., 2009].
In practice, however, complete perfusion protocols are often missing or inconsistent. Time constraints, patient motion, contrast con- traindications, protocol variability, and scanner differences frequently limit acquisition.
This project builds on the observation that, even without perfusion imaging, other MRI se- quences contain physiologically related cues. These can be combined to infer the hypoperfused volume [Oktay et al., 2018]. The main scientific goal is to develop a hetero-modal encoder that integrates any subset of sequences into a unified latent representation, from which a realistic hy- poperfusion map can be reconstructed while preserving anatomical, physical, and inter-sequence consistency.
Problem
Generating coherent hypoperfused maps from heterogeneous MRI inputs presents sev- eral difficulties [Liu et al., 2023]. First, the missing-modality problem arises because patients rarely undergo identical MRI protocols–each case may lack one or more key sequences, such as perfusion- weighted imaging or TOF angiography–making it difficult to learn consistent cross-modal corre- spondences. Second, the domain shift across scanners, vendors, and acquisition settings introduces strong variability in intensity distributions and spatial resolution, which can confound generative models trained on limited datasets. Third, anatomical and physiological consistency must be pre- served: reconstructed hypoperfusion patterns should remain compatible with vascular territories, tissue diffusion constraints, and known hemodynamic principles [Copen et al., 2011]. Finally, data imbalance between normal and ischemic regions complicates loss design and evaluation metrics.
Internship Objectives
To address these issues, the proposed method will rely on a hetero-modal encoder–decoder architecture trained within a variational or diffusion-based generative framework. The encoder will learn a shared latent space constrained by anatomical priors and mutual informa- tion regularization to align representations across modalities.
The objectives are to design and implement a hetero-modal encoder–decoder trained in a vari- ational or diffusion-based generative framework to synthesize hypoperfusion maps from routine MRI. Learn a shared latent space across modalities, constrained by anatomical priors and mutual- information regularization, to align cross-sequence representations. Build robustness to missing sequences and scanner variability via modality dropout and adversarial domain adaptation. Pre- serve vessel detail and global perfusion coherence with multi-scale supervision during decoding. Enforce physiological plausibility by integrating soft constraints (flow continuity, inter-hemispheric symmetry, empiric perfusion–diffusion links).
Application & Expected Impact
Quantify the ischemic ”mismatch” (penumbra) as the vol- ume difference/ratio between perfusion deficit and diffusion core, and assess its clinical utility for reperfusion triage when onset is uncertain and for prognosticating treatment benefit. Deliverables include code, trained models, ablation studies, and validation on retrospective cohorts with report- ing suited for a manuscript draft.
Candidate profile
We look for strongly motivated candidates (i) coming from a math, physics, computer science or engineering diploma (ii) having a strong mathematical background, notably in linear algebra, analysis, probability and statistics, in machine learning and deep learning (iii) having good programming skills on some scientific language, preferably python.
Knowledge of medical imaging, particularly MRI, is not required, but is a strong plus. Knowl- edge of basic optimization theory is also appreciated.
Practical information
The intern will be mainly hosted at the UFR science and technology (40 rue du Pelvoux) close to the city center. However, he/she may also spend some periods at the Hospital of Corbeil.
The monthly internship gratification is of about €670.
Application procedure: send a motivation letter, a CV and your University transcript (relev´e de notes) since 1st year BSc to {vincent.vigneron,hichem.maaref}@univ-evry.fr.
What we offer
Hands-on experience with cutting-edge AI techniques for medical imaging.
Tackle real-world, high-impact healthcare problems using deep learning. Close mentorship from experienced researchers at the IBISC laboratory.
Opportunities to co-author publications and present your work at conferences. Continuation into PhD studies
Contact
{hichem.maaref, vincent.vigneron}@univ-evry.fr,
References
[Copen et al., 2011] Copen, W. A., Schaefer, P. W., and Wu, O. (2011). Mr perfusion imaging in acute ischemic stroke. Neuroimaging Clinics of North America, 21(2):259–283, x.
[Forkert et al., 2013] Forkert, N. D., Kaesemann, P., Treszl, A., Siemonsen, S., Cheng, B., Handels, H., Fiehler, J., and Thomalla, G. (2013). Comparison of 10 ttp and tmax estimation techniques for mr perfusion-diffusion mismatch quantification in acute stroke. American Journal of Neuroradiology, 34:1697– 1703.
[Liu et al., 2023] Liu, C.-F., Hsu, J., Xu, X., Kim, G., Sheppard, S. M., Meier, E. L., Miller, M. I., Hillis,
- E., and Faria, A. V. (2023). Digital 3d brain mri arterial territories atlas. Scientific Data, 10(1):74.
[Mittal et al., 2009] Mittal, S., Wu, Z., Neelavalli, J., and Haacke, E. M. (2009). Susceptibility-weighted imaging: technical aspects and clinical applications. AJNR American Journal of Neuroradiology, 30(2):232–252.
[Oktay et al., 2018] Oktay, O., Schlemper, J., Folgoc, L. L., Lee, M., Heinrich, M., Misawa, K., Mori, K., McDonagh, S., Hammerla, N. Y., Kainz, B., Glocker, B., and Rueckert, D. (2018). Attention u-net: Learning where to look for the pancreas. arXiv preprint arXiv:1804.03999.
- Date de l’appel : 11/11/2025
- Statut de l’appel : Pourvu
- Contacts : Vincent VIGNERON (PR Univ. Évry, IBISC équipe SIAM), Hichem MAAREF (PR IUT Evry, IBISC équipe SIAM), Jean-Philippe CONGE , vincentDOTvigneronATuniv-evryDOTfr, hichemDOTmaarefATuniv-evryDOTfr,
- Sujet de stage niveau Master 2 (format PDF)
- Web équipe SIAM